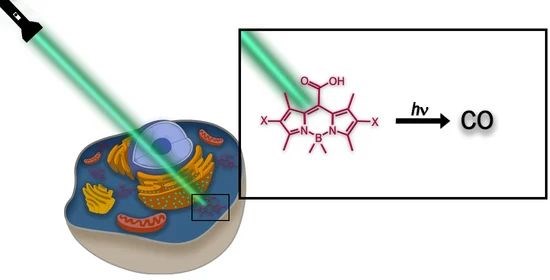
Visible-Light-Activated Carbon Monoxide Release from Porphyrin–Flavonol Hybrids
We report on porphyrin–flavonol hybrids consisting of a porphyrin antenna and four covalently bound 3-hydroxyflavone (flavonol) groups, which act as highly efficient photoactivatable carbon monoxide (CO)-releasing molecules (photoCORMs). These bichromophoric systems enable activation of the UV-absorbing flavonol chromophore by visible light up to 650 nm and offer precise spatial and temporal control of CO administration. The physicochemical properties of the porphyrin antenna system can also be tuned by inserting a metal cation. Our computational study revealed that the process occurs via endergonic triplet–triplet energy transfer from porphyrin to flavonol and may become feasible thanks to flavonol energy stabilization upon intramolecular proton transfer. This mechanism was also indirectly supported by steady-state and transient absorption spectroscopy techniques. Additionally, the porphyrin–flavonol hybrids were found to be biologically benign...

Ramundo A., Janoš J., Muchová L., Šranková M., Vítek L., Slavíček P., Klán P. Visible-Light-Activated Carbon Monoxide Release from Porphyrin-Flavonol Hybrids. J. Am. Chem. Soc. 2024, 146, 920-929.
A Platform for the Synthesis of Oxidation Products of Bilirubin
Bilirubin is the principal product of heme catabolism. High concentrations of the pigment are neurotoxic, yet slightly elevated levels are beneficial. Being a potent antioxidant, oxidative transformations of bilirubin occur in vivo and lead to various oxidized fragments. The mechanisms of their formation, intrinsic biological activities, and potential roles in human pathophysiology are poorly understood. Degradation methods have been used to obtain samples of bilirubin oxidation products for research. Here, we report a complementary, fully synthetic method of preparation. Our strategy leverages repeating substitution patterns in the parent tetracyclic pigment. Functionalized ready-to-couple γ-lactone, γ-lactam, and pyrrole monocyclic building blocks were designed and efficiently synthesized. Subsequent modular combinations, supported by metal-catalyzed borylation and cross-coupling chemistries, translated into the concise assembly of the structurally diverse bilirubin oxidation products (BOXes, propentdyopents, and biopyrrins). The discovery of a new photoisomer of biopyrrin A named lumipyrrin is reported...

Mujawar T., Sevelda P., Madea D., Klán P., Svenda J. A Platform for the Synthesis of Oxidation Products of Bilirubin. J. Am. Chem. Soc. 2024, 146, 1603-1611.
Multimodal Carbon Monoxide Photorelease from Flavonoids
Photooxygenation of flavonoids leads to the release of carbon monoxide (CO). Our structure–photoreactivity study, employing several structurally different flavonoids, including their 13C-labeled analogs, revealed that CO can be produced via two completely orthogonal pathways, depending on their hydroxy group substitution pattern and the reaction conditions. While photooxygenation of the enol 3-OH group has previously been established as the CO liberation channel, we show that the catechol-type hydroxy groups of ring B can predominantly participate in photodecarbonylation.

Ramundo A., Hurtová M., Bożek I., Osifová Z., Russo M., Ngoy B. P., Křen V., Klán P. Multimodal Carbon Monoxide Photorelease from Flavonoids. Org. Lett. 2024, 26, 708-712.
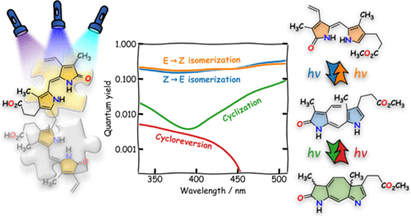
Structure–Photoreactivity Relationship of 3-Hydroxyflavone-Based CO-Releasing Molecules
Carbon monoxide (CO) is an endogenous signaling molecule that regulates diverse physiological processes. The therapeutic potential of CO is hampered by its intrinsic toxicity, and its administration poses a significant challenge. Photoactivatable CO-releasing molecules (photoCORMs) are an excellent tool to overcome the side effects of untargeted CO administration and provide precise spatial and temporal control over its release. Here, we studied the CO release mechanism of a small library of derivatives based on 3-hydroxy-2-phenyl-4H-benzo[g]chromen-4-one (flavonol), previously developed as an efficient photoCORM, by steady-state and femto/nanosecond transient absorption spectroscopies. The main objectives of the work were to explore in detail how to enhance the efficiency of CO photorelease from flavonols, bathochromically shift their absorption bands, control their acid–base properties and solubilities in aqueous solutions, and minimize primary or secondary photochemical side-reactions, such as self-photooxygenation...

Russo M., Orel V., Štacko P., Šranková M., Muchová L., Vítek L., Klán P. Structure-Photoreactivity Relationship of 3-Hydroxyflavone-Based CO-Releasing Molecules. J. Org. Chem. 2022, 87, 4750-4763.
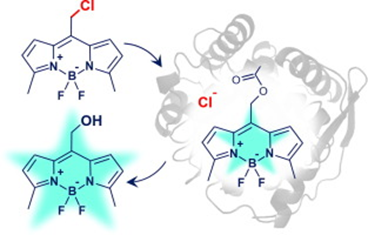
Porphyrin as a Versatile visible-light-activatable organic/metal hybrid photoremovable protecting group
Photoremovable protecting groups (PPGs) represent one of the main contemporary implementations of photochemistry in diverse fields of research and practical applications. For the past half century, organic and metal-complex PPGs were considered mutually exclusive classes, each of which provided unique sets of physical and chemical properties thanks to their distinctive structures. Here, we introduce the meso-methylporphyrin group as a prototype hybrid-class PPG that unites traditionally exclusive elements of organic and metal-complex PPGs within a single structure. We show that the porphyrin scaffold allows extensive modularity by functional separation of the metal-binding chromophore and up to four sites of leaving group release. The insertion of metal ions can be used to tune their spectroscopic, photochemical, and biological properties. We provide a detailed description of the photoreaction mechanism studied by steady-state and transient absorption spectroscopies and quantum-chemical calculations...
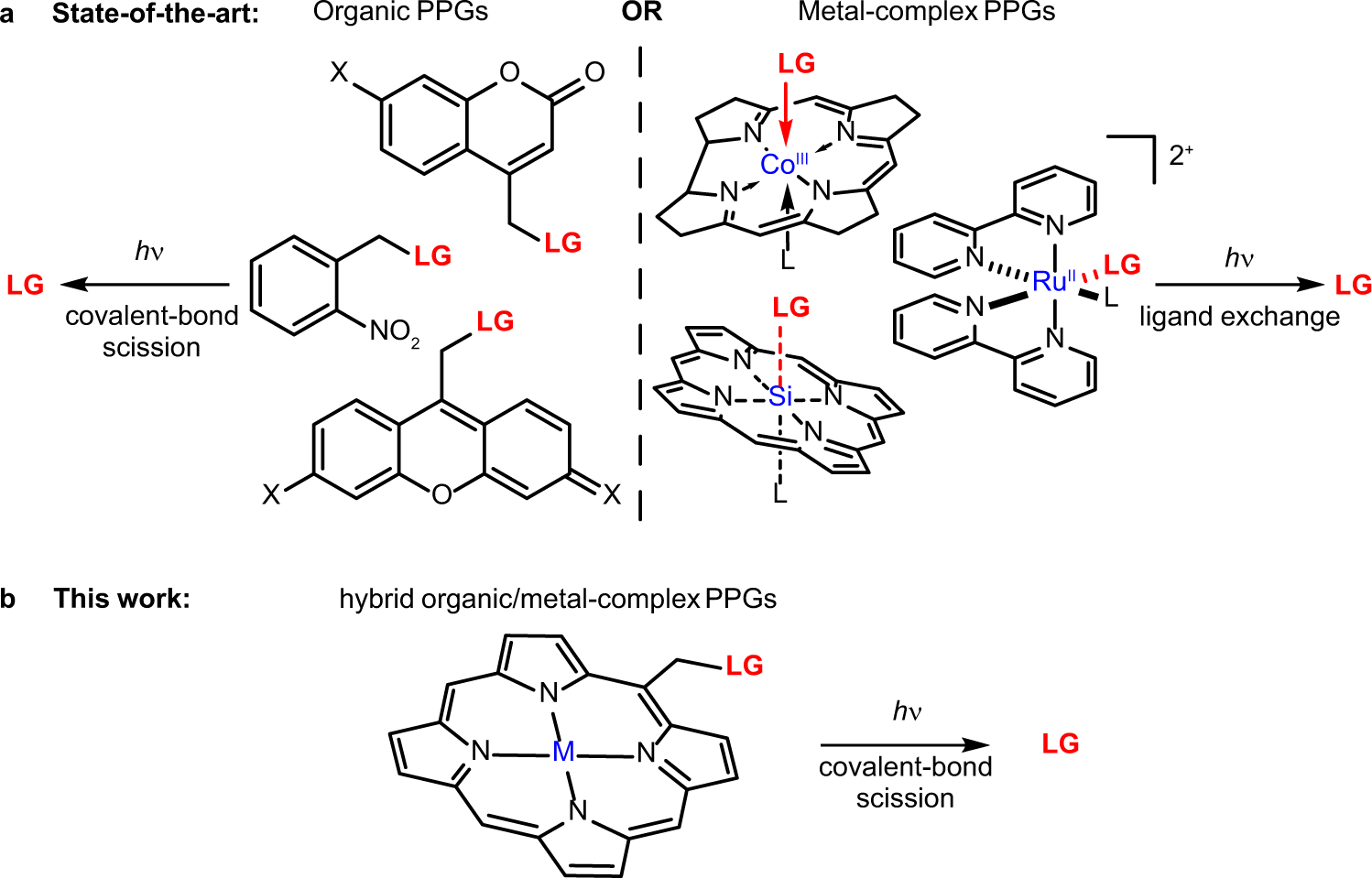
Sekhar A. R., Chitose Y., Janoš J., Dangoor S. I., Ramundo A., Satchi-Fainaro R., Slavíček P., Klán P., Weinstain R. Porphyrin as a Versatile Visible-Light-Activatable Organic/Metal Hybrid Photoremovable Protecting Group. Nat. Commun. 2022, 13, 3614.
Spin–Vibronic Control of Intersystem Crossing in Iodine-Substituted Heptamethine Cyanines
Spin–orbit coupling between electronic states of different multiplicity can be strongly coupled to molecular vibrations, and this interaction is becoming recognized as an important mechanism for controlling the course of photochemical reactions. Here, we show that the involvement of spin–vibronic coupling is essential for understanding the photophysics and photochemistry of heptamethine cyanines (Cy7), bearing iodine as a heavy atom in the C3′ position of the chain and/or a 3H-indolium core, as potential triplet sensitizers and singlet oxygen producers in methanol and aqueous solutions. The sensitization efficiency was found to be an order of magnitude higher for the chain-substituted than the 3H-indolium core-substituted derivatives. Our ab initio calculations demonstrate that while all optimal structures of Cy7 are characterized by negligible spin–orbit coupling (tenths of cm–1) with no dependence on the position of the substituent, molecular vibrations lead to its significant increase (tens of cm–1 for the chain-substituted cyanines), which allowed us to interpret the observed position dependence.

Tovtik R., Muchová E., Štacková L., Slavíček P., Klán P. Spin-Vibronic Control of Intersystem Crossing in Iodine-Substituted Heptamethine Cyanines. J. Org. Chem. 2023, 88, 6716-6728.
Sulfonothioated meso-Methyl BODIPY Shows Enhanced Uncaging Efficiency and Releases H2Sn
meso-Methyl BODIPY photocages stand out for their absorption properties and easy chromophore derivatization. However, their low uncaging efficiencies often hinder applications requiring release of protected substrates in high amounts. In this study, we demonstrate that the sulfonothioated BODIPY group photocleaves a sulfonylthio group from the meso-methyl position with a 10-fold higher quantum yield than the most efficient leaving groups studied to date. Photocleavage, observed in solution and in cells, is accompanied by the spatiotemporally controlled photorelease of H2Sn. For this reason, sulfonothioated BODIPY may be applied in cell signaling, redox homeostasis, and metabolic regulation studies.

Wohlrábová L., Okoročenkova J., Palao E., Kužmová E., Chalupský K., Klán P., Slanina T. Sulfonothioated meso-Methyl BODIPY Shows Enhanced Uncaging Efficiency and Releases H2Sn. Org. Lett. 2023, 25, 6705-6709.
Cyanine Phototruncation Enables Spatiotemporal Cell Labeling
Photoconvertible tracking strategies assess the dynamic migration of cell populations. Here we develop phototruncation-assisted cell tracking (PACT) and apply it to evaluate the migration of immune cells into tumor-draining lymphatics. This method is enabled by a recently discovered cyanine photoconversion reaction that leads to the two-carbon truncation and consequent blue-shift of these commonly used probes. By examining substituent effects on the heptamethine cyanine chromophore, we find that introduction of a single methoxy group increases the yield of the phototruncation reaction in neutral buffer by almost 8-fold. When converted to a membrane-bound cell-tracking variant, this probe can be applied in a series of in vitro and in vivo experiments. These include quantitative, time-dependent measurements of the migration of immune cells from tumors to tumor-draining lymph nodes. Unlike previously reported cellular photoconversion approaches, this method does not require genetic engineering and uses near-infrared (NIR) wavelengths...

Fukushima H., Matikonda S. S., Usama S. M., Furusawa A., Kato T., Štacková L., Klán P., Kobayashi H., Schnermann M. J. Cell Labeling with Spatiotemporal Control Using Cyanine Phototruncation. J. Am. Chem. Soc. 2022, 144, 11075-11080.
Photochemistry of (Z)-Isovinylneoxanthobilirubic Acid Methyl Ester, a Bilirubin Dipyrrinone Subunit: Femtosecond Transient Absorption and Stimulated Raman Emission Spectroscopy
Bilirubin (BR) is an essential metabolite formed by the catabolism of heme. Phototherapy with blue-green light can be applied to reduce high concentrations of BR in blood and is used especially in the neonatal period. In this work, we studied the photochemistry of (Z)-isovinylneoxanthobilirubic acid methyl ester, a dipyrrinone subunit of BR, by steady-state absorption, femtosecond transient absorption, and stimulated Raman spectroscopies. Both the (Z)- and (E)-configurational isomers of isovinylneoxanthobilirubic acid undergo wavelength-dependent and reversible photoisomerization...

Madea D., Mujawar T., Dvořák A., Pospíšilová K., Muchová L., Čubáková P., Kloz M., Švenda J., Vítek L., Klán P. Photochemistry of (Z)-Isovinylneoxanthobilirubic Acid Methyl Ester, a Bilirubin Dipyrrinone Subunit: Femtosecond Transient Absorption and Stimulated Raman Emission Spectroscopy. J. Org. Chem. 2022, 87, 3089-3103.
Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials
Photoactivatable (alternatively, photoremovable, photoreleasable, or photocleavable) protecting groups (PPGs), also known as caged or photocaged compounds, are used to enable non-invasive spatiotemporal photochemical control over the release of species of interest. Recent years have seen the development of PPGs activatable by biologically and chemically benign visible and near-infrared (NIR) light. These long-wavelength-absorbing moieties expand the applicability of this powerful method and its accessibility to non-specialist users. This review comprehensively covers organic and transition metal-containing photoactivatable compounds (complexes) that absorb in the visible- and NIR-range to release various leaving groups and gasotransmitters (carbon monoxide, nitric oxide, and hydrogen sulfide). The text also covers visible- and NIR-light-induced photosensitized release using molecular sensitizers, quantum dots, and upconversion and second-harmonic nanoparticles, as well as release via photodynamic (photooxygenation by singlet oxygen) and photothermal effects. Release from photoactivatable polymers, micelles, vesicles, and photoswitches, along with the related emerging field of photopharmacology, is discussed at the end of the review.
Weinstain R., Slanina T., Kand D., Klán P. Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials. Chem. Rev. 2020, 120, 13135-13272.
Cyanine‐Flavonol Hybrids for Near‐Infrared Light‐Activated Delivery of Carbon Monoxide
Carbon monoxide (CO) is an endogenous signaling molecule that controls a number of physiological processes. To circumvent the inherent toxicity of CO, light‐activated CO‐releasing molecules (photoCORMs) have emerged as an alternative for its administration. However, their wider application requires photoactivation using biologically benign visible and near‐infrared (NIR) light. In this work, a strategy to access such photoCORMs by fusing two CO‐releasing flavonol moieties with a NIR‐absorbing cyanine dye is presented. These hybrids liberate two molecules of CO in high chemical yields upon activation with NIR light up to 820 nm and exhibit excellent uncaging cross‐sections, which surpass the state‐of‐the‐art by two orders of magnitude. Furthermore, the biocompatibility and applicability of the system in vitro and in vivo are demonstrated, and a mechanism of CO release is proposed. It is hoped that this strategy will stimulate the discovery of new classes of photoCORMs and accelerate the translation of CO‐based phototherapy into practice.
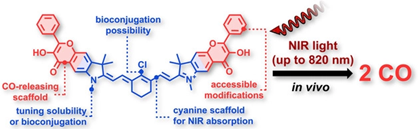
Štacková L., Russo M., Muchová L., Orel V., Vítek L., Štacko P., Klán P. Cyanine-Flavonol Hybrids for Near-Infrared Light-Activated Delivery of Carbon Monoxide. Chem. Eur. J. 2020, 26, 13184-13190.
Deciphering the Structure–Property Relations in Substituted Heptamethine Cyanines
Heptamethine cyanines (Cy7) are fluorophores essential for modern bioimaging techniques and chemistry. Here, we systematically evaluated the photochemical and photophysical properties of a library of Cy7 derivatives containing diverse substituents in different positions of the heptamethine chain. A single substitution allows modulation of their absorption maxima in the range of 693–805 nm and photophysical properties, such as quantum yields of singlet-oxygen formation, decomposition, and fluorescence or affinity to singlet oxygen, within 2–3 orders of magnitude. The same substituent in different positions of the chain often exhibits distinctly contradictory effects, demonstrating that both the type and position of the substituent are pivotal for the design of Cy7-based applications. The combination of experimental results with quantum-chemical calculations provides insights into the structure–property relationship, the elucidation of which will accelerate the development of cyanines with properties tailored for specific applications, such as fluorescent probes and sensors, photouncaging, photodynamic therapy, or singlet-oxygen detection.
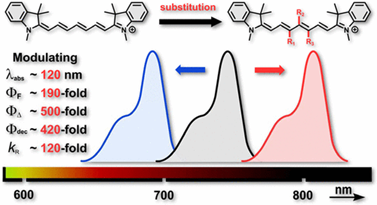
Štacková L., Muchová E., Russo M., Slavíček P., Štacko P., Klán P. Deciphering the Structure–Property Relations in Substituted Heptamethine Cyanines. J. Org. Chem. 2020, 85, 9776-9790.
Conformational Control of the Photodynamics of a Bilirubin Dipyrrinone Subunit: Femtosecond Spectroscopy Combined with Nonadiabatic Simulations
The photochemistry of bilirubin has been extensively studied due to its importance in the phototherapy of hyperbilirubinemia. In the present work, we investigated the ultrafast photodynamics of a bilirubin dipyrrinone subunit, vinylneoxanthobilirubic acid methyl ester. The photoisomerization and photocyclization reactions of its (E) and (Z) isomers were studied using femtosecond transient absorption spectroscopy and by multireference electronic structure theory, where the nonadiabatic dynamics was modeled with a Landau–Zener surface hopping technique.
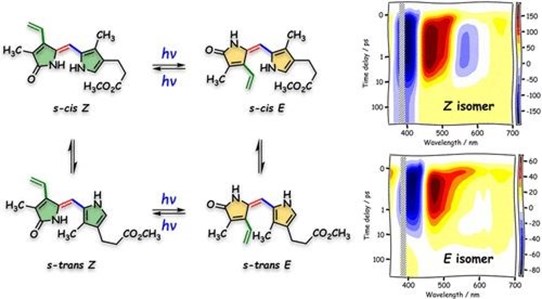
Janoš J., Madea D., Mahvidi S., Mujawar T., Švenda, Jakub; Suchan J., Slavíček P., Klán P. Conformational Control of Photodynamics of Bilirubin Dipyrrinone Subunit: Femtosecond Spectroscopy Combined with Nonadiabatic Simulations. J. Phys. Chem. A 2020, 124, 10457-10471.
Wavelength-Dependent Photochemistry and Biological Relevance of a Bilirubin Dipyrrinone Subunit
Phototherapy is a standard treatment for severe neonatal jaundice to remove toxic bilirubin from the blood. Here, the wavelength-dependent photochemistry of vinylneoxanthobilirubic acid methyl ester, a simplified model of a bilirubin dipyrrinone subunit responsible for a lumirubin-like structural rearrangement, was thoroughly investigated by liquid chromatography and mass and absorption spectroscopies, with the application of a multivariate curve resolution analysis method supplemented with quantum chemical calculations. Irradiation of the model chromophore leads to reversible Z → E photoisomerization followed by reversible photocyclization to a seven-membered ring system (formed as a mixture of diastereomers).
Madea D., Mahvidi S., Chalupa D., Mujawar T., Dvořák A., Muchová L., Janoš J., Slavíček P., Švenda J., Vítek L., Klán P. Wavelength-Dependent Photochemistry and Biological Relevance of a Bilirubin Dipyrrinone Subunit. J. Org. Chem. 2020, 85, 13015-13028.
Effects of Substituents on Photophysical and CO-Photoreleasing Properties of 2,6-Substituted meso-Carboxy BODIPY Derivatives
Carbon monoxide (CO) is an endogenously produced signaling molecule involved in the control of a vast array of physiological processes. One of the strategies to administer therapeutic amounts of CO is the precise spatial and temporal control over its release from photoactivatable CO-releasing molecules (photoCORMs). Here we present the synthesis and photophysical and photochemical properties of a small library of meso-carboxy BODIPY derivatives bearing different substituents at positions 2 and 6. We show that the nature of substituents has a major impact on both their photophysics and the efficiency of CO photorelease. CO was found to be efficiently released from π-extended 2,6-arylethynyl BODIPY derivatives possessing absorption spectra shifted to a more biologically desirable wavelength range. Selected photoCORMs were subjected to in vitro experiments that did not reveal any serious toxic effects, suggesting their potential for further biological research.
Sanchez-Carnerero E. M., Russo M., Jakob A., Muchová L., Vítek L., Klán P. Effects of Substituents on Photophysical and CO-Photoreleasing Properties of meso-Carboxy BODIPY Derivatives. Chemistry 2021, 3, 238-255.
The Impact of Tunnel Mutations on Enzymatic Catalysis Depends on the Tunnel-Substrate Complementarity and the Rate-Limiting Step
Transport of ligands between bulk solvent and the buried active sites is a critical event in the catalytic cycle of many enzymes. The rational design of transport pathways is far from trivial due to the lack of knowledge about the effect of mutations on ligand transport. The main and an auxiliary tunnel of haloalkane dehalogenase LinB have been previously engineered for improved dehalogenation of 1,2-dibromoethane (DBE). The first chemical step of DBE conversion was enhanced by L177W mutation in the main tunnel, but the rate-limiting product release was slowed down because the mutation blocked the main access tunnel and hindered protein dynamics. Three additional mutations W140A + F143L + I211L opened-up the auxiliary tunnel and enhanced the product release, making this four-point variant the most efficient catalyst with DBE. Here we study the impact of these mutations on the catalysis of bulky aromatic substrates, 4-(bromomethyl)-6,7-dimethoxycoumarin (COU) and 8-chloromethyl-4,4′-difluoro-3,5-dimethyl-4-bora-3a,4a-diaza-s-indacene (BDP).
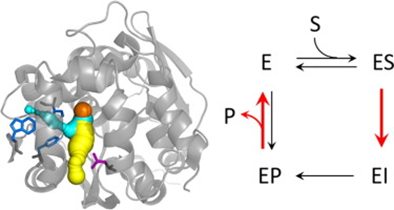
Kokkonen P., Slánská M., Dočkalová V., Pinto G., Sanchez-Carnerero E., Damborský J., Klán P., Prokop Z. The Impact of Tunnel Mutations on Enzymatic Catalysis Depends on the Tunnel-Substrate Complementarity and the Rate-Limiting Step. Comput. Struct. Biotechnol. J. 2020, 18, 805-813.
Fluorescent Substrates for Haloalkane Dehalogenases: Novel Probes for Mechanistic Studies and Protein Labeling
Haloalkane dehalogenases are enzymes that catalyze the cleavage of carbon-halogen bonds in halogenated compounds. They serve as model enzymes for studying structure–function relationships of >100.000 members of the α/β-hydrolase superfamily. Detailed kinetic analysis of their reaction is crucial for understanding the reaction mechanism and developing novel concepts in protein engineering. Fluorescent substrates, which change their fluorescence properties during a catalytic cycle, may serve as attractive molecular probes for studying the mechanism of enzyme catalysis. In this work, we present the development of the first fluorescent substrates for this enzyme family based on coumarin and BODIPY chromophores. Steady-state and pre-steady-state kinetics with two of the most active haloalkane dehalogenases, DmmA and LinB, revealed that both fluorescent substrates provided specificity constant two orders of magnitude higher (0.14–12.6 μM−1 s−1) than previously reported representative substrates for the haloalkane dehalogenase family (0.00005–0.014 μM−1 s−1).
Dočkalová V., Sanchez-Carnerero E., Dunajová Z., Palao E., Slánská M., Buryška T., Damborský J., Klán P., Prokop Z. Fluorescent Substrates for Haloalkane Dehalogenases: Novel Probes for Mechanistic Studies and Protein Labeling. Comput. Struct. Biotechnol. J. 2020, 18, 922-932.
Photochemistry of a 9‐Dithianyl‐Pyronin Derivative: A Cornucopia of Reaction Intermediates Lead to Common Photoproducts
Leaving groups attached to the meso‐methyl position of many common dyes, such as xanthene, BODIPY, or pyronin derivatives, can be liberated upon irradiation with visible light. However, the course of phototransformations of such photoactivatable systems can be quite complex and the identification of reaction intermediates or even products is often neglected. This paper exemplifies the photochemistry of a 9‐dithianyl‐pyronin derivative, which undergoes an oxidative transformation at the meso‐position to give a 3,6‐diamino‐9H‐xanthen‐9‐one derivative, formic acid, and carbon monoxide as the main photoproducts. The course of this multi‐photon multi‐step reaction was studied under various conditions by steady‐state and time‐resolved optical spectroscopy, mass spectrometry and NMR spectroscopy to understand the effects of solvents and molecular oxygen on individual steps. Our analyses have revealed the existence of many intermediates and their interrelationships to provide a complete picture of the transformation, which can bring new inputs to a rational design of new photoactivatable pyronin or xanthene derivatives.
Martínek M., Váňa J., Šebej P., Navrátil R., Slanina T., Ludvíková L., Roithová J., Klán P. Photochemistry of a 9-Dithianyl-Pyronin Derivative: A Cornucopia of Reaction Intermediates Leads to Common Photoproducts. ChemPlusChem 2020, 85, 2230-2242.
Mechanisms of Orthogonal Photodecarbonylation Reactions of 3-Hydroxyflavone-Based Acid–Base Forms
Carbon monoxide is a naturally occurring gasotransmitter combining inherent toxicity with a remarkable therapeutic potential and arduous administration. Photoactivatable carbon monoxide-releasing molecules (photoCORMs) are chemical agents that allow for precise spatial and temporal control over the CO release. In this work, we present a comprehensive mechanistic study of the photochemical CO release from 3-hydroxy-2-phenyl-4H-chromen-4-one, a π-extended 3-hydroxyflavone photoCORM, in methanol using steady-state and transient absorption spectroscopies and quantum chemical calculations. The multiplicity of the productive excited states and the role of oxygen (O2) in the CO production are emphasized, revealing a photoreaction dichotomy of the 3-hydroxyflavone acid and base forms. The utilization of three major orthogonal mechanistic pathways, all of which lead to the CO release, can fuel future endeavors to improve the CO release efficacy of 3-hydroxyflavone-based derivatives and refine their potential medical applications as photoCORMs.
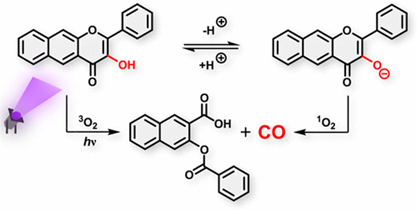
Russo M., Štacko P., Nachtigallová D., Klán P. Mechanisms of Orthogonal Photodecarbonylation Reactions of 3-Hydroxyflavone-Based Acid-Base Forms. J. Org. Chem. 2020, 85, 3527-3537.
Structural Modifications of Nile Red Carbon Monoxide Fluorescent Probe: Sensing Mechanism and Applications
Carbon monoxide (CO) is a cell-signaling molecule (gasotransmitter) produced endogenously by oxidative catabolism of heme, and the understanding of its spatial and temporal sensing at the cellular level is still an open challenge. Synthesis, optical properties, and study of the sensing mechanism of Nile red Pd-based CO chemosensors, structurally modified by core and bridge substituents, in methanol and aqueous solutions are reported in this work. The sensing fluorescence “off–on” response of palladacycle-based sensors possessing low-background fluorescence arises from their reaction with CO to release the corresponding highly fluorescent Nile red derivatives in the final step. Our mechanistic study showed that electron-withdrawing and electron-donating core substituents affect the rate-determining step of the reaction. More importantly, the substituents were found to have a substantial effect on the Nile red sensor fluorescence quantum yields, hereby defining the sensing detection limit.
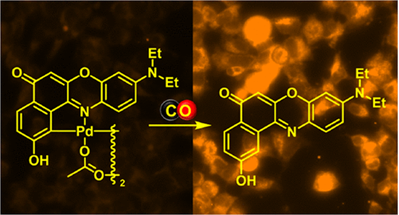
Madea D., Martínek M., Muchová L., Vítek L., Klán P. Structural Modifications of Nile Red Carbon Monoxide Fluorescent Probe: Sensing Mechanism and Applications. J. Org. Chem. 2020, 85, 3473-3489.
Laser Flash Photolysis Study of the Photoinduced Oxidation of 4-(Dimethylamino)benzonitrile (DMABN)
Aromatic amines are aquatic contaminants for which phototransformation in surface waters can be induced by excited triplet states of dissolved organic matter (3DOM*). The first reaction step is assumed to consist of a one-electron oxidation process of the amine to produce its radical cation. In this paper, we present laser flash photolysis investigations aimed at characterizing the photoinduced, aqueous phase one-electron oxidation of 4-(dimethylamino)benzonitrile (DMABN) as a representative of this contaminant class. The production of the radical cation of DMABN (DMABN˙+) after direct photoexcitation of DMABN at 266 nm was confirmed in accord with previous experimental results. Moreover, DMABN˙+ was shown to be produced from the reactions of several excited triplet photosensitizers (carbonyl compounds) with DMABN.
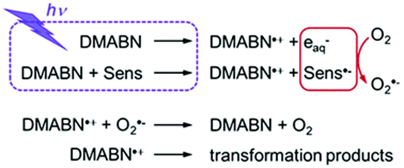
Leresche F., Ludvíkova L., Heger D., Klán P., von Gunten U., Canonica S. Laser Flash Photolysis Study of the Photoinduced Oxidation of 4-(Dimethylamino)benzonitrile (DMABN). Photochem. Photobiol. Sci. 2019, 18, 534-545.
Approach to Substituted Heptamethine Cyanine Chain by Ring Opening of Zincke Salts
Cyanine dyes play an indispensable and central role in modern fluorescence-based biological techniques. Despite their importance and widespread use, the current synthesis methods of heptamethine chain modification are restricted to coupling reactions and nucleophilic substitution at the meso position in the chain. Herein, we report the direct transformation of Zincke salts to cyanine dyes under mild conditions, accompanied by the incorporation of a substituted pyridine residue into the heptamethine scaffold. This work represents the first general approach that allows the introduction of diverse substituents and different substitution patterns at the C3′–C5′ positions of the chain. High yields, functional tolerance, versatility toward the condensation partners, and scalability make this method a powerful tool for accessing a new generation of cyanine derivatives.
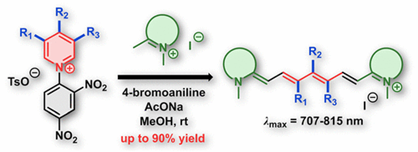
Štacková L., Štacko P., Klán P. Approach to Substituted Heptamethine Cyanine Chain by Ring Opening of Zincke Salts. J. Am. Chem. Soc. 2019, 141, 7155-7162.
The Effect of Light Wavelength on in vitro Bilirubin Photodegradation and Photoisomer Production
The action spectrum for bilirubin photodegradation has been intensively studied. However, questions still remain regarding which light wavelength most efficiently photodegrades bilirubin. In this study, we determined the in vitro effects of different irradiation wavelength ranges on bilirubin photodegradation. In our system, the optimum bilirubin photodegradation and lumirubin production rates occurred between 490 and 500 nm. Spectra shapes were remarkably similar, suggesting that lumirubin production was the major process of bilirubin photodegradation.
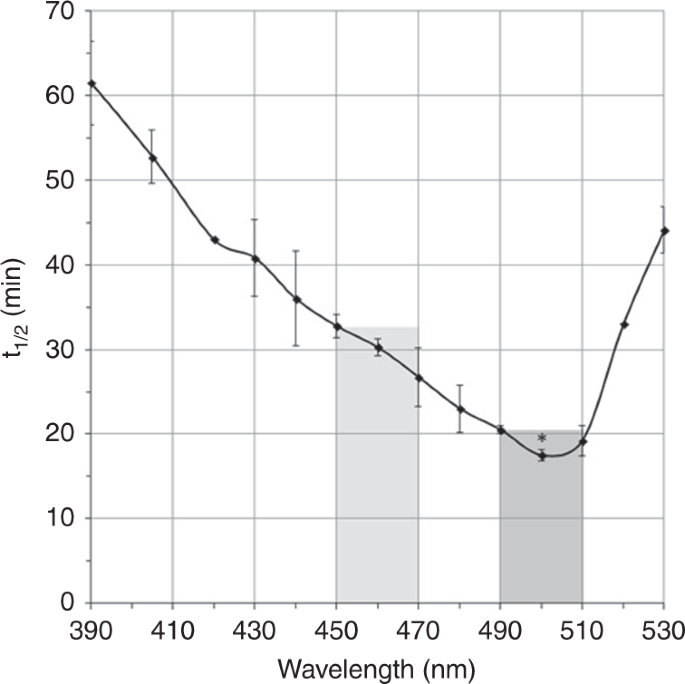
Vreman H. J., Kourula S., Jašprová J., Ludvíková L., Klán P., Muchová L., Vítek L., Cline B. K., Wong R. J., Stevenson D. K. The Effect of Light Wavelength on in vitro Bilirubin Photodegradation and Photoisomer Production. Pediatr. Res. 2019, 85, 865-873.
Fluorescent pH Indicators for Neutral to Near-Alkaline Conditions Based on 9-Iminopyronin Derivatives
Monitoring enzymatic activities at pH ranges compatible with their physiological optimum using fluorescent assays is important for high-throughput screening and engineering of novel biocatalysts as well as applications in biosensing and diagnostics. However, the number of fluorescent dyes exhibiting necessary optical properties at alkaline pHs is limited. Here, we report the design, synthesis, and biochemical application of fluorescent 9-iminopyronin derivatives possessing unique and distinct emission properties to provide optimal sensitivity at neutral to near-alkaline conditions. They exist in two acid–base forms, allowing for a dual-emission ratiometric sensing with a linear response of pH between 6.0 and 8.5 and detection sensitivity of a 0.06 pH unit. Selected fluorophores were used as the sensors for monitoring the enzymatic hydrolytic activity of a model enzyme haloalkane dehalogenase.
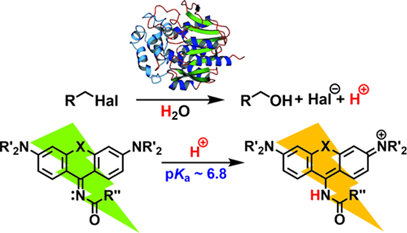
Horváth P., Šebej P., Kovář D., Damborský J., Prokop Z., Klán P. Fluorescent pH Indicators for Neutral to Near Alkaline Conditions Based on 9-Iminopyronin Derivatives. ACS Omega 2019, 4, 5479-5485.
Photosensitized Cross-Linking of Tryptophan and Tyrosine Derivatives by Rose Bengal in Aqueous Solutions
Photosensitized cross-linking of N-acetyl derivatives of tryptophan and tyrosine by rose bengal in phosphate buffer saline was studied by steady-state absorption and emission spectroscopy, nanosecond transient absorption spectroscopy, and chemical analyses. These amino acids undergo cross-linking to form dimeric and higher oligomeric products under anoxic conditions with rose bengal as a sacrificial oxidant, whereas they react predominantly with singlet oxygen produced by rose bengal in aerated solutions. This investigation provides a comprehensive view into the first steps of rose bengal photosensitized cross-linking of these two amino acids.
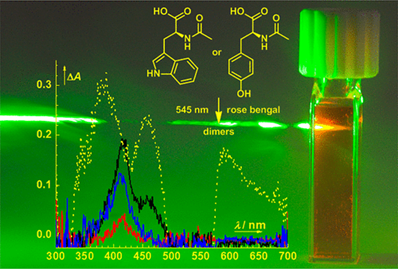
Ludvíková L., Štacko P., Sperry J., Klán P. Photosensitized Crosslinking of Tryptophan and Tyrosine Derivatives by Rose Bengal in Aqueous Solutions. J. Org. Chem. 2018, 83, 10835-10844.
Visible to NIR Light Photoactivation of Hydrogen Sulfide for Biological Targeting
The synthesis and photochemical properties of H2S-releasing BODIPY thiocarbamate photocage scaffolds activatable by visible-to-NIR (up to 700 nm) light to release carbonyl sulfide (COS), which is transformed to H2S using either isolated or natural carbonic anhydrase, is reported. The excellent uncaging cross section and high H2S release yields in in vitro experiments, including live-cell imaging, suggest that these photocages can serve as a platform for the bio-orthogonal phototriggered release within the tissue-transparent window.
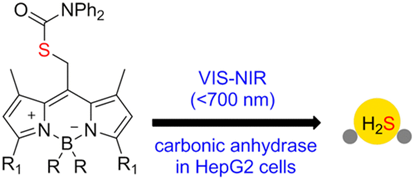
Štacko P., Muchová L., Vítek L., Klán P. Visible to NIR Light Photoactivation of Hydrogen Sulfide for Biological Targeting. Org. Lett. 2018, 20, 4907-4911.
Protected 5-(Hydroxymethyl)uracil Nucleotides Bearing Visible-Light Photocleavable Groups as Building Blocks for Polymerase Synthesis of Photocaged DNA
Nucleosides, nucleotides and 2′-deoxyribonucleoside triphosphates (dNTPs) containing 5-(hydroxymethyl)uracil protected with photocleavable groups (2-nitrobenzyl-, 6-nitropiperonyl or 9-anthrylmethyl) were prepared and tested as building blocks for the polymerase synthesis of photocaged oligonucleotides and DNA. Photodeprotection (photorelease) reactions were studied in detail on model nucleoside monophosphates and their photoreaction quantum yields were determined. Photocaged dNTPs were then tested and used as substrates for DNA polymerases in primer extension or PCR.
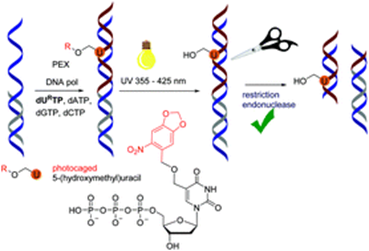
Boháčová S., Ludvíková L., Poštová Slavětínská L., Vaníková Z., Klán P., Hocek M. Protected 5-(Hydroxymethyl)uracil Nucleotides Bearing Photocleavable Groups as Building Blocks for Polymerase Synthesis of Photocaged DNA. Org. Biomol. Chem. 2018, 16, 1527-1535.
In Search of the Perfect Photocage: Structure-Reactivity Relationships in meso-Methyl BODIPY Photoremovable Protecting Groups
A detailed investigation of the photophysical parameters and photochemical reactivity of meso-methyl BODIPY photoremovable protecting groups was accomplished through systematic variation of the leaving group (LG) and core substituents as well as substitutions at boron. Efficiencies of the LG release were evaluated using both steady-state and transient absorption spectroscopies as well as computational analyses to identify the optimal structural features. We find that the quantum yields for photorelease with this photocage are highly sensitive to substituent effects....
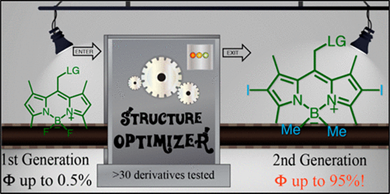
Slanina T. , Shrestha P., Palao E., Kand D., Peterson J., Dutton A., Rubinstein N., Weinstain R., Winter A., Klán P. In Search of the Perfect Photocage: Structure-Reactivity Relationships in meso-Methyl BODIPY Photoremovable Protecting Groups. J. Am. Chem. Soc. 2017, 139, 15168-15175.
Controlled Photorelease of Alkynoic Acids and Their Decarboxylative Deprotection for Copper-Catalyzed Azide/Alkyne Cycloaddition
A controlled photorelease of alkynoic acids from the meso-methyl BODIPY photoremovable protecting group facilitates their subsequent efficient decarboxylation to give terminal alkynes for a CuI-catalyzed azide/alkyne cycloaddition. The quantum efficiencies of the photochemical step and the kinetics of the click reaction step are reported.
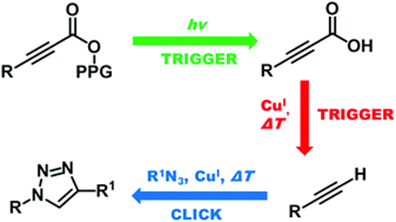
Vohradská N., Sánchez-Carnerero, E., Pastierik T., Mazal C., Klán P. Controlled Photorelease of Alkynoic Acids and their Decarboxylative Deprotection for Copper-Catalyzed Azide/Alkyne Cycloaddition. Chem. Commun. 2018, 54, 5558-5561.
Photooxidation of Aniline Derivatives Can Be Activated by Freezing Their Aqueous Solutions
A combined experimental and computational approach was used to investigate the spectroscopic properties of three different aniline derivatives (aniline, N,N-dimethylaniline, and N,N-diethylaniline) in aqueous solutions and at the air–ice interface in the temperature range of 243–298 K. The absorption and diffuse reflectance spectra of ice samples prepared by different techniques, such as slow or shock freezing of the aqueous solutions or vapor deposition on ice grains, exhibited unequivocal bathochromic shifts of 10–15 nm of the absorption maxima of anilines in frozen samples compared to those in liquid aqueous solutions. DFT and SCS-ADC(2) calculations showed that contaminant–contaminant and contaminant–ice interactions are responsible for these shifts....
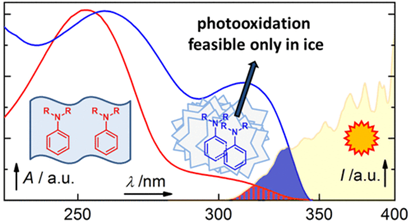
Corrochano P., Nachtigallová D., Klán P. Photooxidation of Aniline Derivatives Can Be Activated by Freezing Their Aqueous Solutions. Environ. Sci. Technol. 2017, 51, 13763-13770.
Transition-Metal-Free CO-Releasing BODIPY Derivatives Activatable by Visible to NIR Light as Promising Bioactive Molecules
Carbon monoxide-releasing molecules (CORMs) are chemical agents used to administer CO as an endogenous, biologically active molecule. A precise spatial and temporal control over the CO release is the major requirement for their applications. Here, we report the synthesis and properties of a new generation of transition-metal-free carbon monoxide-releasing molecules based on BODIPY chromophores (COR-BDPs) activatable by visible-to-NIR (up to 730 nm) light. We demonstrate their performance for both in vitro and in vivo experimental settings, and we propose the mechanism of the CO release based on steady-state and transient spectroscopy experiments and quantum chemical calculations.
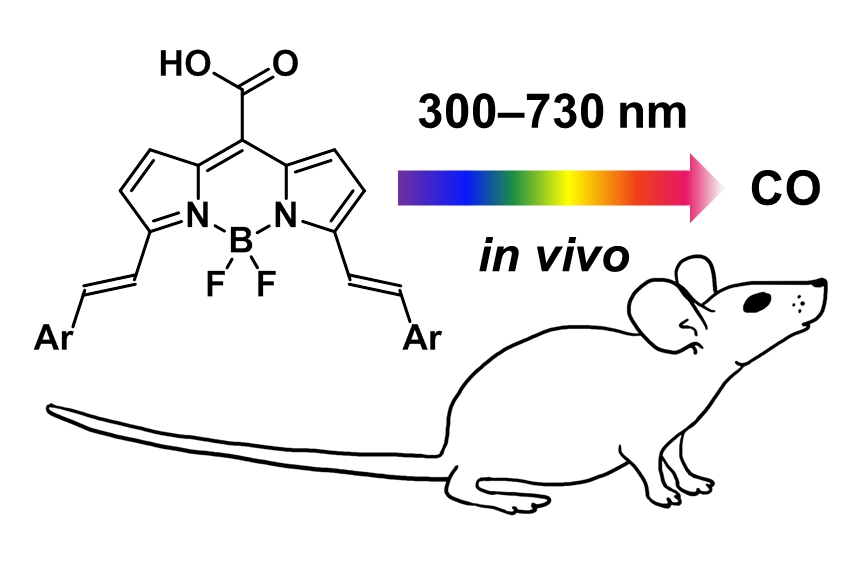
Palao E., Slanina T., Muchová L., Šolomek T., Vítek L., Klán P. Transition-Metal-Free CO-Releasing BODIPY Derivatives Activatable by Visible to NIR Light as Promising Bioactive Molecules. J. Am. Chem. Soc. 2016, 138, 126-133.
Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy
Photoremovable protecting groups (PPGs) release molecules
such as enzymes, neurotransmitters, signaling molecules, fluorophores, insecticides, pheromones, and fragrances that thereby exhibit desirable physical, chemical, or biological qualities upon photoactivation. They are synthetically malleable and accessible, offering a wide range of structures for designed applications. They offer excellent spatial and temporal control for the substrate release. Their applications span many scientific fields, from DNA chip technology, drug delivery, and photoregulation of proteins, to rheology, solid-phase synthesis, surface chemistry, and nanotechnology. PPGs are excellent, versatile tools for time-resolved studies of chemical processes in living cells. Multiphoton excitation can provide superior spatial resolution, and the new chromophores included herein offer more precise temporal control for addressing the dynamics of in vivo events in living organisms.
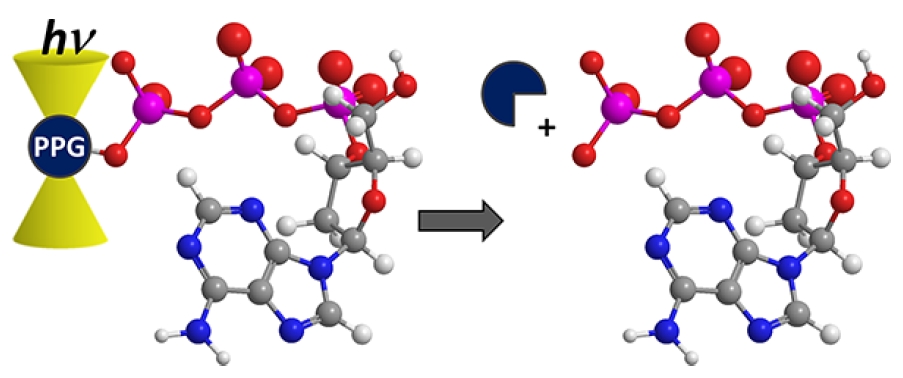 Klán P., Šolomek T., Bochet C., Blanc A., Givens R. S., Rubina M., Popik V., Kostikov A., Wirz J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119-191.
Klán P., Šolomek T., Bochet C., Blanc A., Givens R. S., Rubina M., Popik V., Kostikov A., Wirz J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119-191.
Bambusuril as a One-Electron Donor for Photoinduced Electron Transfer to Methyl Viologen in Mixed Crystals
Methyl viologen hexafluorophosphate (MV2+·2PF6–) and dodecamethylbambus[6]uril (BU6) form crystals in which the layers of viologen dications alternate with those of a 1:2 supramolecular complex of BU6 and PF6–. This arrangement allows for a one-electron reduction of MV2+ ions upon UV irradiation to form MV+• radical cations within the crystal structure with half-lives of several hours in air. The mechanism of this photoinduced electron transfer in the solid state and the origin of the long-lived charge-separated state were studied by steady-state and transient spectroscopies, cyclic voltammetry, and electron paramagnetic resonance spectroscopy. Our experiments are supported by quantum-chemical calculations showing that BU6 acts as a reductant.
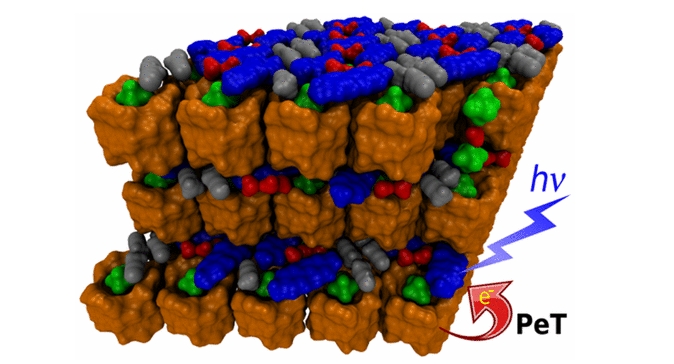
Fiala T., Ludvíková L., Heger D., Švec J., Slanina T., Vetráková Ľ., Babiak M., Nečas M., Kulhánek P., Klán P., Šindelář V. Bambusuril as a One Electron Donor for Photoinduced Electron Transfer to Methyl Viologen in Mixed Crystals. J. Am. Chem. Soc. 2017, 139, 2597-2603.
Small-Molecule Fluorophores with Large Stokes Shifts: 9-Iminopyronin Analogues as Clickable Tags
The design, synthesis, and both experimental and theoretical studies of several novel 9-(acylimino)- and 9-(sulfonylimino)pyronin derivatives containing either an oxygen or a silicon atom at position 10 are reported. These compounds, especially the Si analogues, exhibit remarkably large Stokes shifts (around 200 nm) while still possessing a high fluorophore brightness, absorption bands in the near-UV and visible part of the spectrum, and high thermal and photochemical stabilities in protic solvents. The reason for the observed large Stokes shifts is an intramolecular charge-transfer excitation of an electron from the HOMO to the LUMO of the chromophore, accompanied by elongation of the C9–N bond and considerable solvent reorganization due to hydrogen bonding to the solvent. Due to the photophysical properties of the studied compounds and their facile and high-yielding synthesis, as well as a simple protocol for their bioorthogonal ligation to a model saccharide using a Huisgen alkyne–azide cycloaddition, they represent excellent candidates for biochemical and biological applications as fluorescent tags and indicators for multichannel imaging.
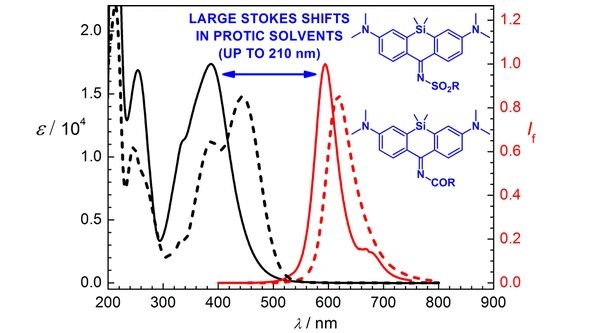
Horváth P., Šebej P., Šolomek T., Klán P. Small-Molecule Fluorophores with Large Stokes Shifts: 9-Iminopyronin Analogues as Clickable Tags. J. Org. Chem. 2015, 80, 1299-1311.
Spectroscopic Properties of Anisole at the Air–Ice Interface: A Combined Experimental–Computational Approach
A combined experimental and computational approach was used to investigate the spectroscopic properties of anisole in aqueous solutions and at the ice–air interface in the temperature range of 77–298 K. The absorption, diffuse reflectance, and emission spectra of ice samples containing anisole prepared by different techniques to evaluate changes in the contaminated ice matrix that occur at different temperatures. It was found that the position of the lowest absorption band of anisole and its tail shift bathochromically by ∼4 nm in frozen samples compared to liquid aqueous solutions. On the other hand, the emission spectra of aqueous anisole solutions were found to fundamentally change upon freezing. DFT and ADC(2) calculations were used to interpret the absorption and emission spectra of anisole monomer and dimer associates.
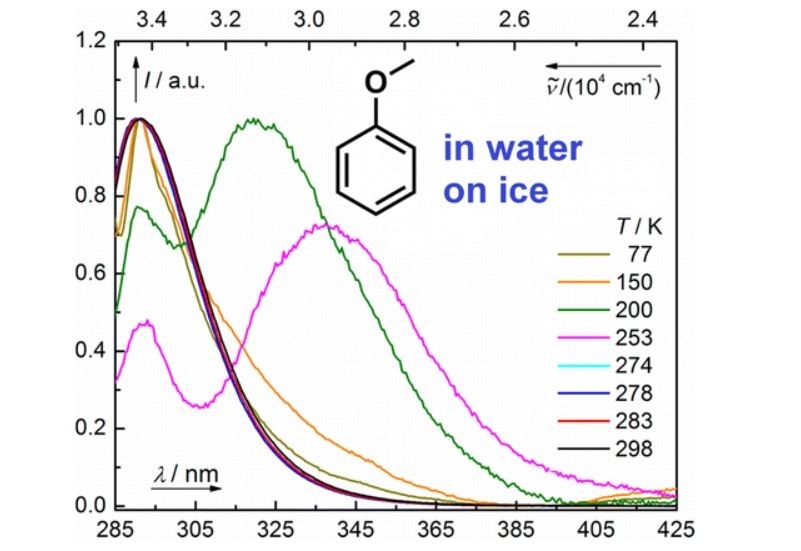
K'Ekuboni Malongwe J., Nachtigallová D., Corrochano P., Klán P. Spectroscopic Properties of Anisole at the Air-Ice Interface: A Combined Experimental-Computational Approach. Langmuir 2016, 32, 5755-5764.
Photoswitching of Azobenzene-Based Reverse Micelles above and at Subzero Temperatures As Studied by NMR and Molecular Dynamics Simulations
We designed and studied the structure, dynamics, and photochemistry of photoswitchable reverse micelles (RMs) composed of azobenzene-containing ammonium amphiphile 1 and water in chloroform at room and subzero temperatures by NMR spectroscopy and molecular dynamics simulations. The NMR and diffusion coefficient analyses showed that micelles containing either the E or Z configuration of 1 are stable at room temperature. Depending on the water-to-surfactant molar ratio, the size of the RMs remains unchanged or is slightly reduced because of the partial loss of water from the micellar cores upon extensive E → Z or Z → E photoisomerization of the azobenzene group in 1. Upon freezing at 253 or 233 K, E-1 RMs partially precipitate from the solution but are redissolved upon warming whereas Z-1 RMs remain fully dissolved at all temperatures. Light-induced isomerization of 1 at low temperatures does not lead to the disintegration of RMs remaining in the solution; however, its scope is influenced by a precipitation process. To obtain a deeper molecular view of RMs, their structure was characterized by MD simulations. It is shown that RMs allow for amphiphile isomerization without causing any immediate significant structural changes in the micelles.
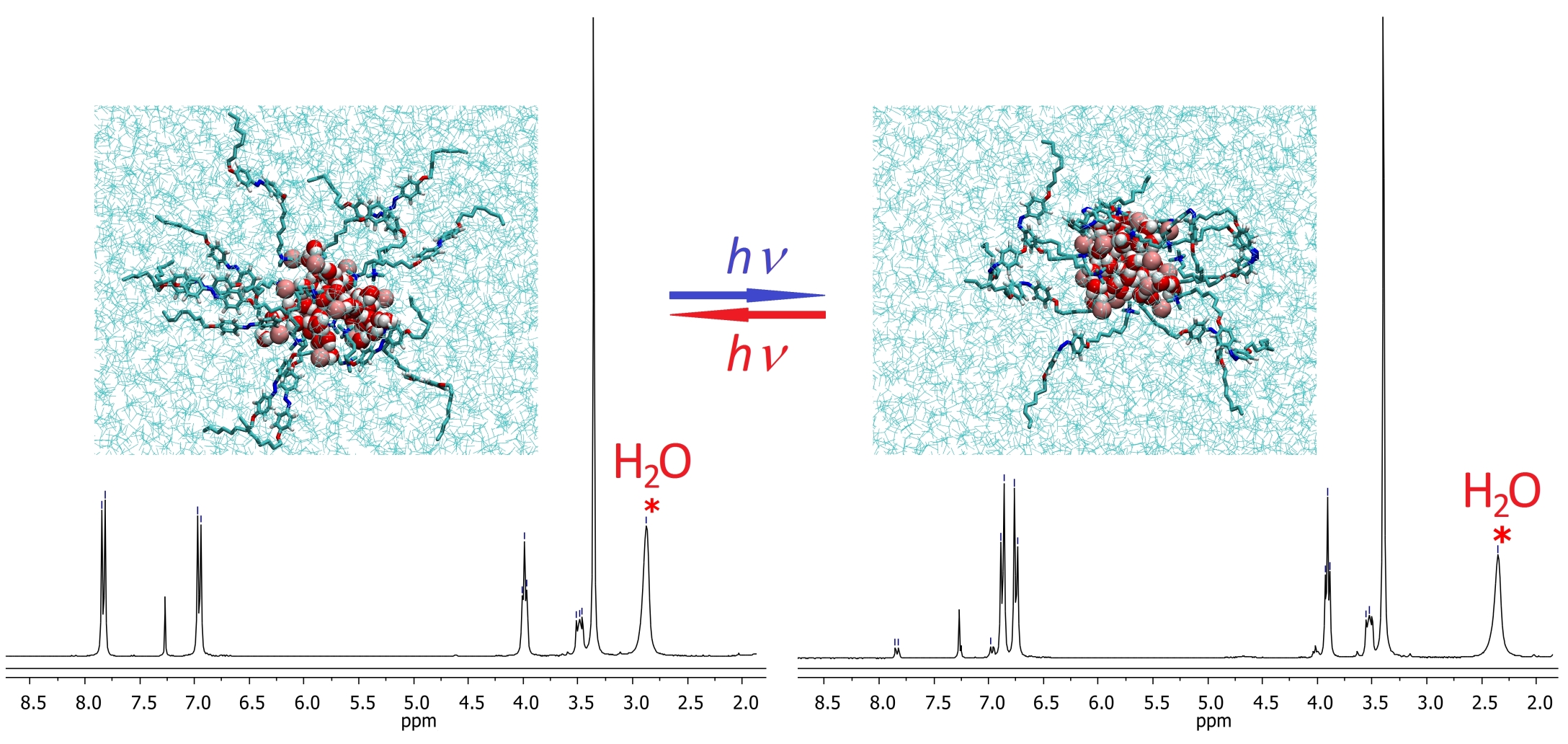
Filipová L., Kogagen M., Štacko P., Muchová E., Slavíček P., Klán P. Photoswitching of Azobenzene-Based Reverse Micelles above and at Subzero Temperatures As Studied by NMR and Molecular Dynamics Simulations. Langmuir 2017, 33, 2306-2317.
Photochemistry of Rose Bengal in Water and Acetonitrile: A Comprehensive Kinetic Analysis
The photophysical and photochemical properties of rose bengal (RB) in degassed aqueous and acetonitrile solutions were studied using steady-state and transient absorption spectroscopies. This comprehensive investigation provides detailed information about the kinetics and the optical properties of all intermediates involved: the triplet excited state and the oxidized and reduced forms of RB. A full kinetic description is used to control the concentrations of these intermediates by changing the initial experimental conditions.
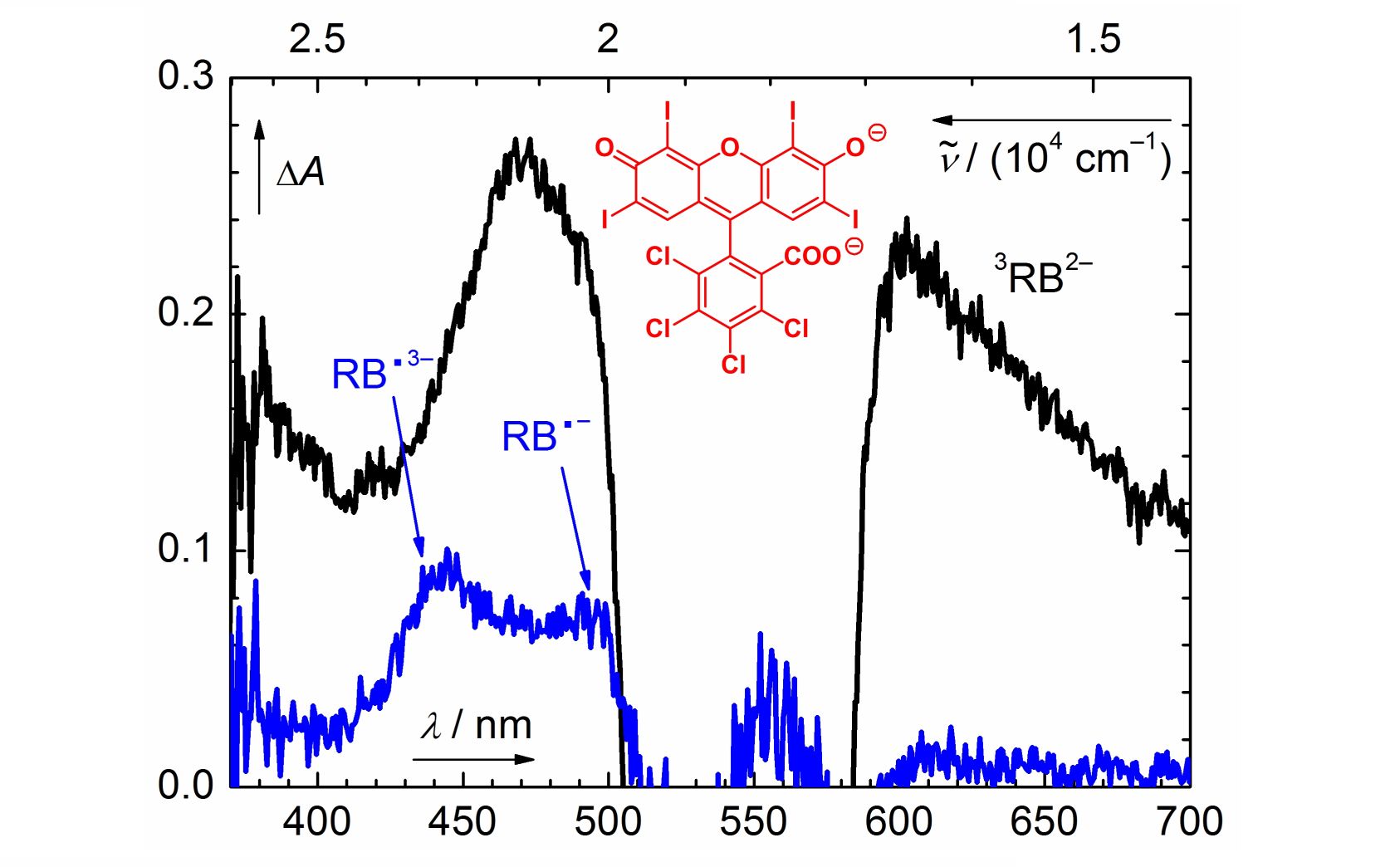
Ludvíková L., Friš P., Heger D., Wirz J., Šebej P., Klán P. Photochemistry of Rose Bengal in Water and Acetonitrile: A Comprehensive Kinetic Analysis. Phys. Chem. Chem. Phys. 2016, 18, 16266-16273.
Searching for Improved Photoreleasing Abilities of Organic Molecules
Photoremovable protecting groups (PPGs) are chemical auxiliaries that provide spatial and temporal control over the release of various molecules: bioagents (neurotransmitters and cell-signaling molecules, Ca2+ ions), acids, bases, oxidants, insecticides, pheromones, fragrances, etc. A major challenge for the improvement of PPGs lies in the development of organic chromophores that release the desired bioagents upon continuous irradiation at wavelengths above 650 nm, that is, in the tissue-transparent window. Understanding of the photorelease reaction mechanisms, investigated by laser flash photolysis and rationalized with the aid of quantum chemical calculations, allows for achieving this goal.
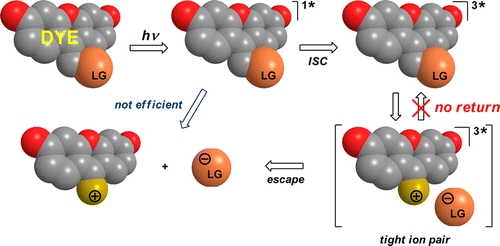
Šolomek T., Wirz J., Klán P. Searching for Improved Photoreleasing Abilities of Organic Molecules. Acc. Chem. Res. 2015, 48, 3064-3072.
A ‘Photorelease, Catch and Photorelease’ Strategy for Bioconjugation Utilizing a p-Hydroxyphenacyl Group
A bioorthogonal ‘catch and photorelease’ strategy, which combines alkyne–azide cycloaddition between p-hydroxyphenacyl azide and alkyne derivatives to form a 1,2,3-triazole adduct and subsequent photochemical release of the triazole moiety via a photo-Favorskii rearrangement, is introduced. The first step can also involve photorelease of a strained alkyne and its Cu-free click reaction with azide.
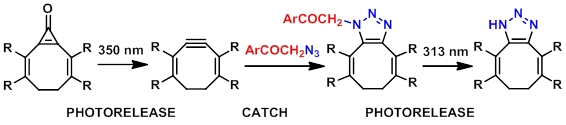
Madea D., Slanina T., Klán P. ‘Photorelease, Catch and Photorelease’ Strategy for Bioconjugation Utilizing p-Hydroxyphenacyl Group. Chem. Commun. 2016, 52, 12901-12904.
Construction of the Carbon–Chalcogen (S, Se, Te) Bond at the 2,6-Positions of BODIPY via Stille Cross-Coupling Reaction
Seven new 2-chalcogen- or 2,6-dichalcogen- (S, Se, Te) BODIPY derivatives were synthesized in good to excellent yields (55–95%) by a Pd-catalyzed C–heteroatom Stille cross-coupling reaction, overcoming the limitations of SNAr. The fluorophores show interesting tunable optical properties associated with the formation of a twisted intramolecular charge transfer excited state and competing intersystem crossing.
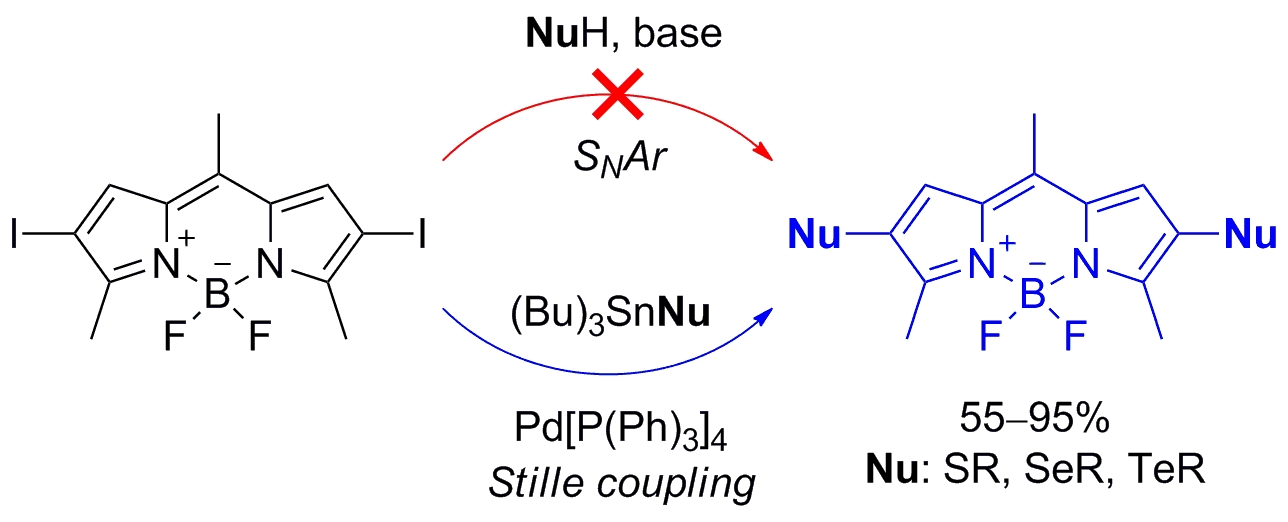
Palao E., Slanina T., Klán P. Construction of the Carbon−Chalcogen (S, Se, Te) Bond at the 2,6-Positions of BODIPY via Stille Cross-Coupling Reaction. Chem. Commun. 2016, 52, 11951-11954.
Photochemical Formation of Dibenzosilacyclohept-4-yne for Cu-Free Click Chemistry with Azides and 1,2,4,5-Tetrazines
Photochemical generation of dibenzosilacyclohept-4-yne 3 from the corresponding cyclopropenone 1 and its copper-free click reactions are reported. Steady-state irradiation, kinetic, and transient absorption spectroscopy studies revealed that strained alkyne 3 is rapidly (<5 ns) and efficiently (Φ = 0.58–0.71) photoreleased from 1 and undergoes remarkably fast, selective, and high-yielding 1,3-dipolar cycloaddition with benzyl azide (∼20 M–1 s–1) or [4 + 2] inverse-electron-demand Diels–Alder reaction with 1,2,4,5-tetrazines (∼260 M–1 s–1) in both methanol and acetonitrile.
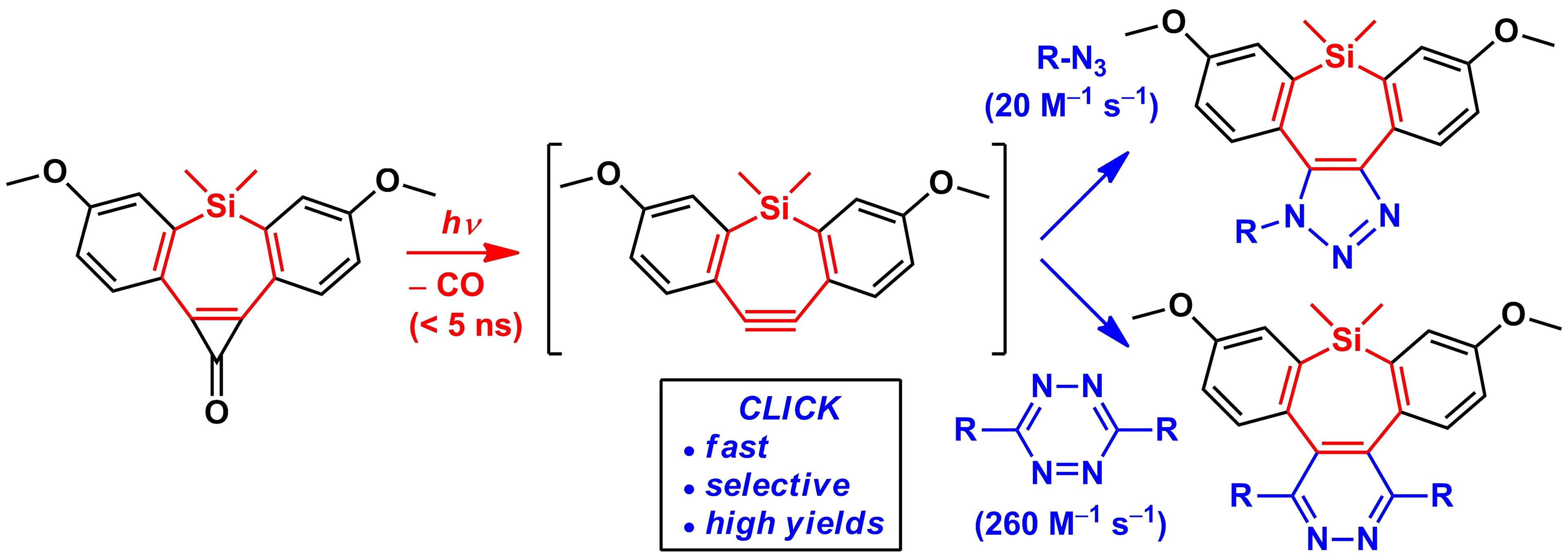
Martínek M., Filipová L., Galeta J., Ludvíková L., Klán P. Photochemical Formation of Dibenzosilacyclohept-4-yne for Cu-Free Click Chemistry with Azides and 1,2,4,5-Tetrazines. Org. Lett. 2016, 18, 4892-4895.
Near-Infrared Fluorescent 9-Phenylethynylpyronin Analogues for Bioimaging
The syntheses and biological applications of two novel fluorescent 9-phenylethynylpyronin analogues containing either carbon or silicon at the position 10 are reported. Both fluorescent probes exhibited a relatively strong fluorescence in methanol and phosphate buffer saline in the near-infrared region (705–738 nm) upon irradiation of either of their absorption maxima in the blue and red regions. The compounds showed high selectivity toward mitochondria in myeloma cells in vivo and allowed their visualization in a favored tissue-transparent window, which makes them promising NIR fluorescent tags for applications in bioimaging.
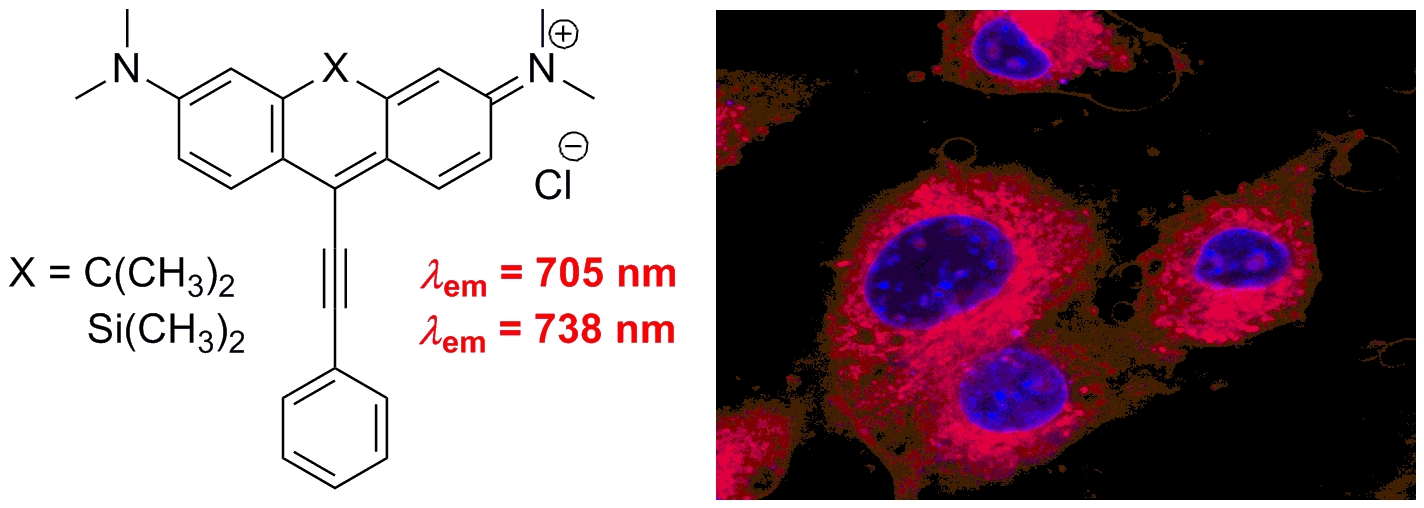
Pastierik T., Šebej P., Medalová J., Štacko P., Klán P. Near-Infrared Fluorescent 9-Phenylethynylpyronin Analogues for Bioimaging. J. Org. Chem. 2014, 79, 3374-3382.
